The branch of biology that studies the genomes of tumours is known as Cancer Genomics. Before explaining what Cancer Genomics is, let’s look at the basic structures of our bodies: the cells.

The DNA of our cells accumulates errors or mutations naturally during our life.
What are mutations and what effects do they have? What can cause them?
Cancer arises through the subsequent accumulation of cancer driver mutations that confer cells with an advantage to proliferate over its neighbours.
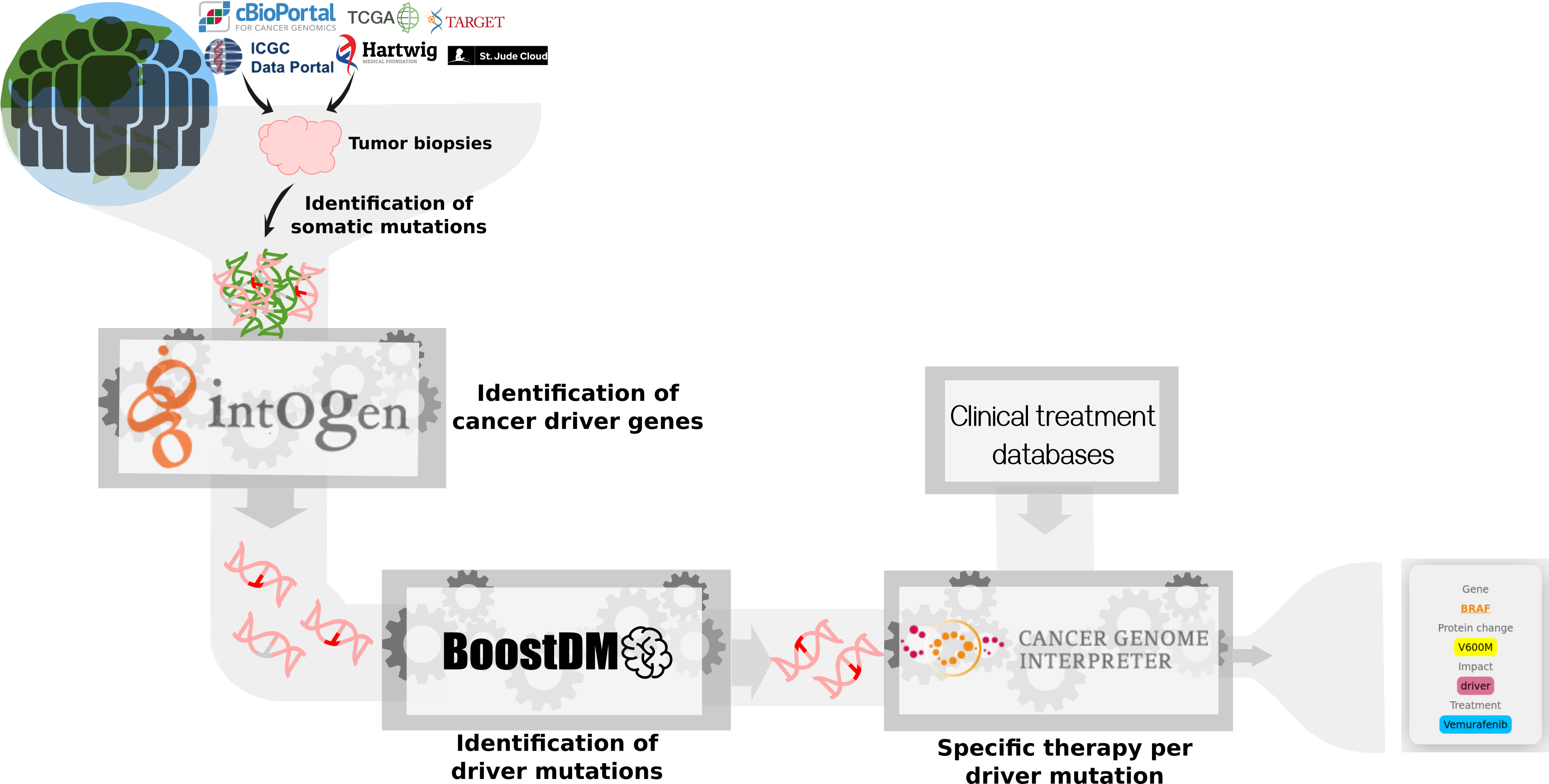
The genomic alterations found in the tumors of cancer patients are different. Identifying cancer drivers in each patient can help to provide better treatments.
Finding cancer drivers is a challenge. Cancers have from 100 to 100,000 of mutations, but we know that only a small percentage of them are cancer drivers. So how can we find them?